Abstract
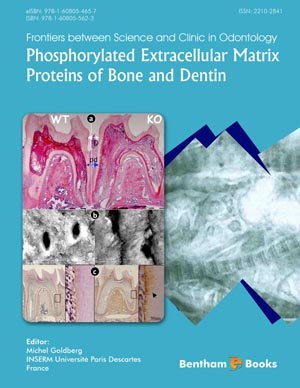
Bone is composed of a mineralized matrix synthesized by osteoblastic cells and maintained by osteocytic cells embedded within this calcified environment. This review of recent literature reveals that regulation of bone formation is influenced by a complex panoply of secreted factors and cell surface receptors which control proliferation and differentiation of osteoproprogenitor cells as well as the functional activity and survival of osteoblastic cells and osteocytes. The osteocyte, the mechanosensor of bone, plays a prominent regulatory role through its control of osteoblastic lineage cells and of osteoclastic lineage cells in remodeling of bone in response to external mechanical forces. This concept is illustrated with specific examples of local, paracrine and systemic signaling mechanisms mediating the responses of cells in bone. As an indication of the inherent specificity of intercellular communication mediated by osteocytes, sclerostin and FGF23, a local and a systemic effector secreted by osteocytes, respectively, are also used as molecular and functional biomarkers. Sclerostin expression by osteocytes is inversely related to mechanical stimulation and sclerostin binds to the Lrp5/6 receptor on osteoblastic cells to inhibit the Wnt1/β-catenin signaling pathway controlling bone formation. Recent work has also shown that the viability of osteocytes is influenced by estrogen deficiency since ovariectomy in sheep increased apoptosis or programmed cell death. The mechanism whereby osteocytes regulate osteoclastogenesis was recently clarified using osteopetrotic RANKL null mice. Osteocytes were shown to be the major source for this required pro-osteoclastogenic factor displaying a greater capacity to support osteoclastogenesis than osteoblasts or bone stromal cells. Finally, while the proliferative actions of growth factors and cytokines are well documented, TGF-β appears to also play a prominent role in the prevention of apoptosis of osteoblastic cells. As illustrated above, the response of bone cells to a generic signal can lead to stimulation or suppression of proliferation of osteoprogenitor cells, to increased differentiation of bone forming osteoblastic cells, or to regulated apoptosis of osteoblasts and/or osteocytes.
Keywords: Osteoblasts, osteocytes, mechanosensors, sclerostin, FGF23, Wnt/beta-catenin, estrogen deficiency, RANKL null mice, growth factors, PHEX, BMP2, BMP7, cytokines, apoptosis.